 Just a couple years ago, I was a research associate working at McGill University in the Meakins-Christie Laboratories, studying a rare disease called lymphangioleiomyomatosis, or LAM. LAM is a progressive, cystic disease afflicting young women with noncancerous lung tumors that can destroy lung function, making the disease potentially fatal. My job was to understand where these tumors came from and what made them propagate throughout the lungs. There was one unfortunate caveat: no one had been able to grow LAM tumor cells outside of the body. As anyone who has ever worked with cancer biology can attest to, there are a multitude of immortalized cancer cell lines, grown from the cells of a patient’s tumor, that can be studied to perform pre-clinical translational research. And yet, not a single representative cell line was available for LAM. Thankfully, my supervisor set me up with just the right project to help solve this puzzle, which centered around induced pluripotent stem cells (iPSCs).
Just a couple years ago, I was a research associate working at McGill University in the Meakins-Christie Laboratories, studying a rare disease called lymphangioleiomyomatosis, or LAM. LAM is a progressive, cystic disease afflicting young women with noncancerous lung tumors that can destroy lung function, making the disease potentially fatal. My job was to understand where these tumors came from and what made them propagate throughout the lungs. There was one unfortunate caveat: no one had been able to grow LAM tumor cells outside of the body. As anyone who has ever worked with cancer biology can attest to, there are a multitude of immortalized cancer cell lines, grown from the cells of a patient’s tumor, that can be studied to perform pre-clinical translational research. And yet, not a single representative cell line was available for LAM. Thankfully, my supervisor set me up with just the right project to help solve this puzzle, which centered around induced pluripotent stem cells (iPSCs).
iPSCs as a new way to model disease
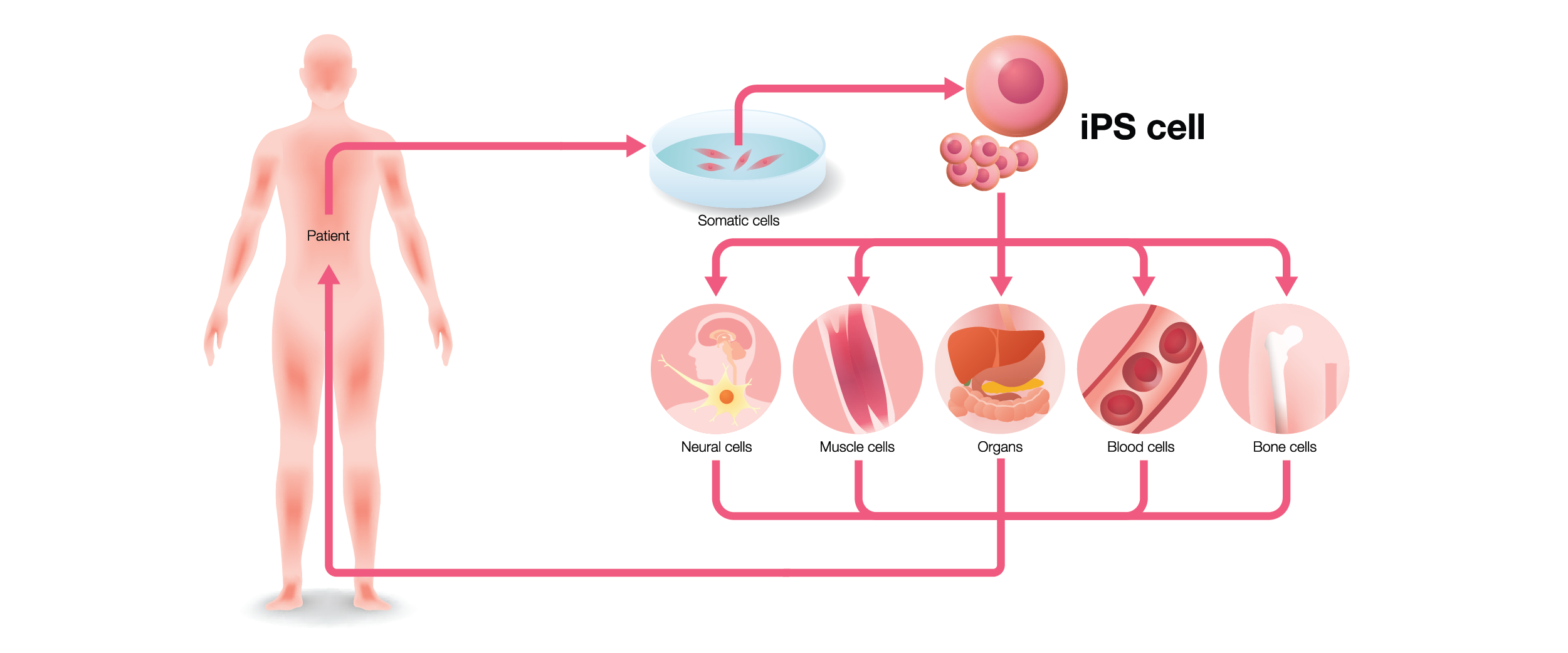
When I began my project, I was set up with a collaborator who introduced me to iPSCs. iPSCs were first engineered in 2007 as a novel method of generating pluripotent cells—that is, they can differentiate into any kind of cell type.1 The only previous source of pluripotent cells was embryonic stem cells (ESCs) derived from blastocysts.2 However, ESCs are relatively difficult to harvest, especially from human subjects, and provide an ethical quandary as the embryo goes to waste once the stem cells are harvested.1 So, what makes iPSCs different? Imagine taking differentiated cells from your body, like blood cells or skin cells, and reverting them back into a pluripotent state. Shinya Yamanaka, who won a Nobel prize for his discovery, did just this by introducing four transcription factors into fibroblasts (KLF-4, SOX2, c-MYC, and OCT4), producing cells that could be differentiated into any other cell type.1,3
iPSCs cells have some obvious advantages over ESCs. You don’t need to harvest blastocysts to generate them, which makes them much more accessible and removes all ethical concerns about working with stem cells. The more interesting benefit to iPSCs is that the tissues used to generate them can be taken directly from the source of the disease, even when the patient is diagnosed later in life. For instance, iPSCs can be generated from patients with a sporadic disease (e.g. Alzheimer’s disease), and the cells can be differentiated back into previously inaccessible cell types, like neural progenitors and neurons, with the same genetic background as the patient.3 In my case, we were able to take some tumors from a patient with LAM, engineer iPSCs with them, then differentiate them back into the cell type LAM tumors most resemble: smooth muscle cells. We even saw typical characteristics of LAM cells using our iPSC model, such as increased invasiveness, low mechanistic target of rapamycin (mTOR) signaling, and neural crest markers common to most LAM cells.4 Because iPSCs can be derived directly from patients, they can also be used to personalize medical approaches, as these cells can, in theory, be tested for the effectiveness of a given pharmaceutical therapy. Finally, using CRISPR-based gene editing technologies, scientists can create mutated iPSC lines, which can then be differentiated into any tissue type while still carrying the mutation of interest.5
Widening the scope of iPSC-based research
iPSCs are quickly becoming one of the primary models used to study human diseases, particularly since animal models usually have a hard time recapitulating the full spectrum of pathological characteristics of human illness.3 iPSCs are currently being used in a variety of fields, including:
Organoid modeling – With the right conditions, organoids—3D aggregates of cells that organize into structures resembling human tissues—can be generated using iPSCs. So far, iPSC-derived organoids exist for neuronal, gastrointestinal, liver, kidney, lung, and cardiac tissues, each able to be screened for therapeutic drugs.6
Drug screening – Because iPSCs can be scaled up to generate a high quantity of cells, they’re ideal candidates for pharmacological screens. Not only can they be used to find drugs that target specific proteins or pathways, but they can also be tested with compounds that might affect things like the production of a specific protein or cell morphology, otherwise known as phenotypic screening. Furthermore, they can be used to test both the efficacy and safety of drugs. Drug safety is particularly important, as preliminary testing with iPSC models could reveal early on that a drug might have serious adverse effects, thus avoiding a failed clinical trial due to drug toxicity.5
Infection and immunity – Most microbiology and immunology studies depend on animal models, like mice, which limits the interpretation of results, as the effects of microbes and the treatments used to eliminate them are not always applicable to humans. Using iPSC-based technology allows these bacterial- and viral-host interactions to be studied in a more relevant model, especially for pathogenic organisms known to only affect humans. iPSCs have already been employed to study the pathophysiology of infection with common microorganisms, such as HIV, Zika virus, H. pylori, and Salmonella.6
Regenerative medicine – iPSCs can generate cells that can be transplanted to treat injured or diseased tissues. One of the advantages of using iPSCs in regenerative medicine is that these cells are derived from the patient, making it less likely that they’ll be rejected. They’re previously been used to treat blood cell loss as well as blindness due to retinal degeneration. By genetically modifying the cells, they can also be used to correct severe genetic diseases, like Duchenne muscular dystrophy.7
Though iPSCs have existed for over 10 years, there’s still a lot that needs to be refined. It’s still relatively expensive to culture and differentiate these cells, as the growth media alone can cost up to 10 times more than your average bottle. Often, you need a “picking hood” in your lab—a microscope within a biosafety cabinet that’s kept sterile and will allow you to pick off spontaneously differentiated cells, helping keep the population of iPSCs undifferentiated and pluripotent. Picking cells can also be a time-consuming process (aiming your pipette tip just right to eliminate those differentiated cells can sure strain your eyes). But the benefits to science and medicine far outweigh these limitations, as iPSCs are in a position to lead the way towards a safer, more personalized way of developing therapies, making them one of the hottest new biological technologies of the last decade.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- Saito-Diaz K, Zeltner N. Induced pluripotent stem cells for disease modeling, cell therapy and drug discovery in genetic autonomic disorders: a review. Clin Auton Res. 2019:1-17.
- Dakhore S, Nayer B, Hasegawa K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018:1-17.
- Farkhondeh A, Li R, Gorshkov K, Chen KG et al. Induced pluripotent stem cells for neural drug discovery. Drug Discov Today. 2019:1-8.
- Julian LM, Delaney SP, Wang Y, et al. Human pluripotent stem cell–derived TSC2-haploinsufficient smooth muscle cells recapitulate features of lymphangioleiomyomatosis. Cancer Res. 2017;77(20):5491-5502.
- Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: A decade of progress. Nat Rev Drug Discov. 2017;16(2):2017.
- Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019:1-12.
- Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015:1-18.


