 This week in our Research Profiles series, we spotlight Emma Jones, a Ph.D. student in the laboratory of Dr. Summer B. Thyme, who studies the mechanisms of neurodevelopmental disorders, such as autism and schizophrenia.
This week in our Research Profiles series, we spotlight Emma Jones, a Ph.D. student in the laboratory of Dr. Summer B. Thyme, who studies the mechanisms of neurodevelopmental disorders, such as autism and schizophrenia.
Zebrafish to Study Neurodevelopment
In Ms. Jones’ lab, they use zebrafish to study neurodevelopment by knocking out panels of genes using the CRISPR/Cas9 gene editing system. The genes chosen are primarily evolutionarily conserved homologs of genes associated with human neurological diseases, many without a previously characterized role in neural development. Once knocked out, Ms. Jones and her colleagues then analyze the phenotypes of the mutants by observing structural brain characteristics and behavior, in addition to performing transcriptomic analysis via RNA sequencing (RNA-seq).
Though distantly related from humans, Ms. Jones stated that studying neurological development in zebrafish has many advantages: “Zebrafish have a fast life cycle, so we are able to look at much larger sample sizes, which have higher statistical power than smaller rodent studies. Zebrafish are also more convenient: they require less space, are cheaper to maintain, and have transparent embryos for imaging. For these reasons, zebrafish are ideal candidates as a model for development, neural or otherwise.”
“I want to contribute to the body of research that will eventually help people like me improve their quality of life.”
Primary Research Interests
Ms. Jones’ primary research project revolves around studying the phenotype of zebrafish lacking a gene unambiguously associated with schizophrenia, meaning that certain mutations—in this case, single nucleotide polymorphisms or SNPs—in this gene are found in many people with schizophrenia. This gene encodes a transcriptional repressor expressed in the brain, inhibiting the expression of a variety of transcripts throughout development. Though her project is in its infancy, she’s hoping to develop a fully formed hypothesis regarding how this gene modulates neural processes and development, following up on some of her large RNA-seq and single-cell sequencing datasets. “My PI’s post-doc work, while she was in the Schier Lab at Harvard, consisted of knocking out 132 zebrafish orthologs of schizophrenia-associated diseases, where she identified over 30 genes of interest. My chosen gene has mutants with different single-cell RNA expression profiles than wild type zebrafish and has small structural decreases in the forebrain. Many of the genes missing in these single-cell clusters are involved in two major processes, stress and social behavior, which are associated with autism and schizophrenia.”
Though Ms. Jones is interested in ultimately finding potential treatments for neurological disorders caused by dysregulated gene expression, her focus right now is understanding the biology of neural development. “It would be relatively straightforward to run a drug screen on specific zebrafish mutants to try and rescue the normal phenotype. Personally, I would like to continue studying neural development in the context of psychiatric disorders and dig deeper into their biological mechanisms. Finding therapies would probably be my next focus, as most major papers end up finding potential therapies for the mechanisms behind disease because finding mechanisms reveals potential therapeutic targets.”
Knock-Ins and Brainbow Mice
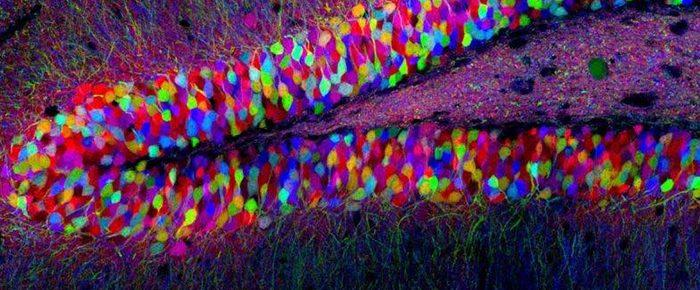
As a secondary project, Ms. Jones is attempting to make more efficient gene knock-ins in zebrafish. She’s doing this by creating RNA-guided transposases, or transposase proteins/complexes with Cas proteins, to insert the gene of interest into the zebrafish genome. “Currently, I am knocking in GFP into zebrafish as proof of concept, without any therapeutic approaches, since we don’t know if it works yet. If we get one of these methods to work, we will optimize it further, making it more efficient and specific, before attempting anything else.” She also has a great appreciation for other models of neurodevelopment, specifically the brainbow mice, one of the most picturesque animal models in all of life science. “The concept of brainbow mice is that neurons are made to randomly express a ratio of red, green, or blue fluorescent proteins, so you end up with 9 different colors expressed throughout the brain. The technique was developed to track and distinguish individual neurons and is used in fields like connectomics.”
Personal Motivation
Ms. Jones shares a deep personal connection to her studies, herself diagnosed with a neurological disorder as a teenager. In high school, her condition made it hard to perform basic school work, so she was referred to a psychiatrist, who tested a variety of therapeutics and psychotherapy until she found a cocktail that allowed her to not just function normally, but thrive enough to land herself in a neuroscience Ph.D. program at the University of Alabama at Birmingham. Though Ms. Jones still continually struggles with depression, she hopes that a career in neuroscience might one day lead to a better life for herself and those around her. “I have personal and familial experience with conditions such as mood disorders and developmental disorders, so I know how devastating and cumbersome they can be. I want to contribute to the body of research that will eventually help people like me improve their quality of life.”
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.




