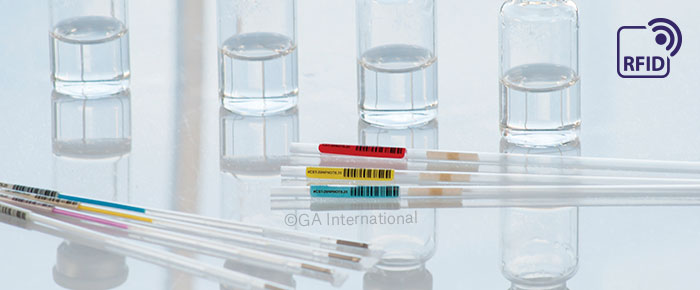
Artificial reproduction technology (ART) facilities and in vitro fertilization (IVF) clinics rely on a host of measures to prevent mix-ups from occurring. The most common tool used is the electronic witnessing system (EWS), which forces technicians to scan each sample as its processed, ensuring the proper eggs and sperm as used to generate embryos. Many of these systems rely on barcodes; however, new radio-frequency identification (RFID) technology has the ability to revolutionize EWS functionality, providing an upgrade that enhances tracking and traceability, while reducing the potential for errors.
What is RFID?
RFID works with long-range radiowave or microwave signals to convey information. To utilize RFID technology, you need a reader, encoder, and RFID tags or labels. There are active RFID tags, which continually emit a signal, or passive tags, which require the reader to emit an initial signal for the tag to send one back.
Using RFID comes with several advantages. Unlike traditional barcodes, RFID labels and tags do not require direct line-of-sight to be read, and multiple tags can be read simultaneously. RFID chips can also store more data than barcodes, while providing additional security if needed.
How is RFID used in an EWS?
One of the key challenges in IVF labs is guaranteeing the parental identity of the fetus. With so many egg and sperm samples banked in large storage facilities, it’s easy to mix them up and select the wrong parental lineage. These mistakes aren’t cheap either; many parents aren’t shy about suing the clinic responsible when their baby does not share their genetic makeup. EWS is a system that helps prevent these mix-ups by forcing technicians to scan each sample as its processed, thereby clearly distinguishing the correct parents and identifying errors early on.
An EWS can rely on barcode labels, RFID labels, or both for sample identification. Systems that rely solely on barcodes require users to manually scan labels affixed to cryo straws and dishes throughout each process. RFID systems are more complex and provide additional features that limit errors. Firstly, because RFID technology has advanced to the point where nearly any label can be provided with an RFID inlay, cryogenic labels for cryo straws and vitrification devices can now be outfitted with RFID. This is especially crucial for cryo straws, as only specialized labels that are low in volatile organic compounds (VOCs) can be used. With RFID-integrated cryo straws, you can simply remove a rack from the liquid nitrogen Dewars and allow the user to determine the number and identity of samples accrued in storage.
When eggs, sperm, or embryos are removed and processed, RFID tags are encoded with the appropriate patient information, including identities, processing date, cell numbers, and volume. A convenient feature of RFID not available with barcodes is that RFID tags and labels can be encoded to emit a signal whenever they are in close proximity to a sample from any donor that is not the correct one, sending an alert to the technician to halt the procedure. This is especially helpful for embryogenesis, where it’s imperative not to use incorrect gametes accidentally.
Note that barcodes can be used in conjunction with RFID labels, as any label outfitted with RFID can also be printed with 1D or 2D barcodes. This can be accomplished using a single printer, with barcodes serving as a backup measure in an RFID-based EWS.
Real-world experience
One study by Gupta et al. in 2020 detailed how an IVF lab integrated an RFID-based EWS. In their study, it took one week to implement the RFID system and another week to train doctors, clinical embryologists, operating theater nurses, and staff personnel. Throughout the study, no errors or mismatches were made that could be characterized as true errors. There were also no significant differences between groups in fertilization or embryo development.1
Another study, headed by Forte et al. in 2016, used the IVF Witness system to assess patient perspectives and feelings towards using an RFID-based EWS. Around 90% of patients initially expressed concerns regarding sample mix-ups occurring in the IVF facility. Implementation of the RFID system alleviated these concerns in 92% of patients, 97% of which were satisfied with the electronic traceability of gametes and embryos. Overall, 97% of patients were comfortable with an IVF lab equipped with an RFID-based EWS.2
RFID is a constantly evolving technology; as such, it is now capable of integrating into nearly every type of application, including all IVF and assisted reproduction technology procedures. Its adaptability makes it ideal in preventing personnel from working with the wrong sperm, eggs, and embryos, ensuring mix-ups are caught before processing and maintaining the integrity of IVF labs for years to come.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
Reference:
- Gupta S, et al. A Preliminary Experience of Integration of an Electronic Witness System, its Validation, Efficacy on Lab Performance, and Staff Satisfaction Assessment in a Busy Indian in vitro Fertilization Laboratory. J Hum Reprod Sci. 2020;13(4):333-339.
- Forte M, et al. Electronic witness system in IVF—patients perspective. J Assist Reprod Genet. 2016;33:1215–1222.