 Using RNA interference (RNAi) as a basic research tool to knock down gene expression is one of the staples of life science research. It’s used in many cellular models, primarily as a tool for assessing the involvement of specific genes in biological systems. However, it wasn’t until recently that this technology was successfully adapted for humans to treat disease.
Using RNA interference (RNAi) as a basic research tool to knock down gene expression is one of the staples of life science research. It’s used in many cellular models, primarily as a tool for assessing the involvement of specific genes in biological systems. However, it wasn’t until recently that this technology was successfully adapted for humans to treat disease.
RNAi – Discovery & Basic Research Tool
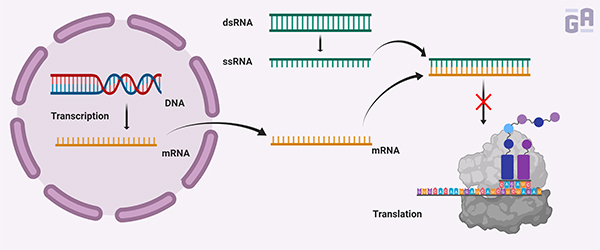
The discovery of RNAi dates back more than 20 years ago. While a few researchers were able to show that exogenously expressed genes could repress the endogenous expression of genes with homologous sequences, it was Fire and Mello that illustrated how double-stranded RNA (dsRNA) could specifically silence gene expression. By treating C. elegans with either single-stranded RNA (ssRNA) or dsRNA, they found that dsRNA was consistently more effective than ssRNA at reducing mRNA levels.1 It was this elegant series of experiments that led Fire and Mello to receive the Nobel Prize in Physiology or Medicine in 2006.2
In 2001, three years after its initial characterization, RNAi was described in mammalian cells for the first time by Thomas Tuschl using exogenous reporter genes from sea pansies and fireflies. The technology was quickly adapted to animals in 2003, with small interfering RNAs (siRNAs) targeting the Fas gene shown to protect mice from hepatitis, increasing the survival rate from 0%—with death occurring due to acute liver failure—to 82%. As a sign of things to come, they noted that up to 90% of the injected siRNAs accumulate specifically in liver cells.1
How siRNA works
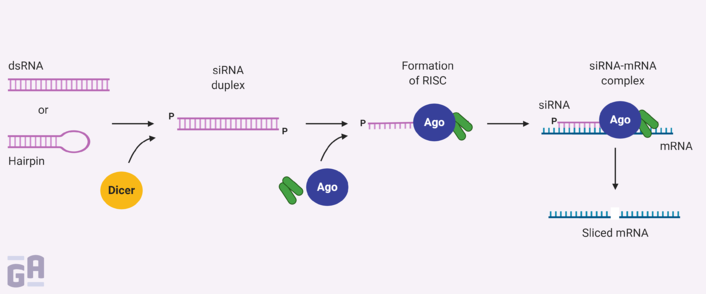
siRNAs are composed of two 21-base oligonucleotide strands with 19 complementary bases and an overhang of 2 nucleotides at each 3ʹ end. Transfecting siRNAs results in their accumulation in the cytoplasm, where the endoribonuclease Dicer separates the double-stranded RNA, with whichever strand has the more stable 5ʹ end integrating into an active RNA-inducing silencing complex (RISC). The single-stranded RNA then guides the RISC to find the correct mRNA and cut it up using the catalytic subunit of the RISC, Ago2.1,2
Since its initial discovery, around 100,000 articles have been published on PubMed.1 Many of these have used siRNA more as a biological tool for investigative research rather than as a potential therapeutic. It can be used in many cell culture-based assays to transiently knock down gene expression, allowing scientists to assess the involvement of specific mRNAs and proteins in their system of choice. In vitro, small hairpin RNA (shRNA) technology evolved from this concept, which consists of 80 base pairs and a region that folds into a hairpin. These can be integrated into plasmid vectors to generate siRNAs inside cells, allowing researchers to develop models that stably knock down gene expression long-term.
 First foray into the clinic
First foray into the clinic
The first ever human clinical trial with siRNA wasn’t Alnylam’s groundbreaking ONPATTRO (patisiran); it was an siRNA against ribonucleotide reductase (RRM2), an enzyme used in nucleic acid metabolism, delivered using polymer nanoparticles to treat solid tumors.1 In the phase I trial, titled CALAA-1 (NCT00689065), cancer patients were injected with the siRNA, with tumors biopsied and measured for the absorption of the drug. Though the drug effectively accumulated inside tumor cells and reduced the levels of RRM2 mRNA, the trial was discontinued due to a high incidence of adverse events.3
Several years later, in 2018, ONPATTRO officially became the first FDA-approved siRNA-based therapeutic to hit the market. Alnylam put in 16 years’ worth of research and $2.5 billion to finally get their drug approved for patients with hereditary transthyretin (hATTR) amyloidosis. hATTR amyloidosis is a genetic disease that manifests as symptoms affecting both the heart and the nervous system and is caused by the deposition of misfolded transthyretin proteins. Mutations in the TTR gene result in a misfolded TTR tetramer, which aggregates into fibrils that stick to nerves and other cells, and ultimately results in the death of the patient.1 ONPATTRO, a slightly modified TTR siRNA delivered via lipid nanoparticles composed of DLin-MC3-DMA, passed all its tests in the APOLLO phase III trial (NCT01960348), reducing the levels of TTR protein by 80% and significantly improving the reflexes, strength, sensation, nerve conduction, postural blood pressure, and overall quality of life of patients with hATTR amyloidosis.2
Upcoming RNAi-based therapies
Alnylam already has another siRNA-based therapy on the market with aminolevulinate synthase 1 (ALAS1) siRNA, GIVLAARI (givosiran), which was approved for the treatment of adults with acute hepatic porphyria. Unlike ONPATTRO, GIVLAARI uses a different delivery method, relying on N-Acetyl-D-galactosamine (GalNAc) to transport the siRNA into liver cells via asialoglycoprotein receptor-mediated and clathrin-involved endocytosis. Alnylam’s profile also includes ongoing clinical trials for products aimed at treating hypercholesterolemia, primary hyperoxaluria, proteinuria, hemoglobinuria, alpha-1 liver disease, and hypertension, many of which are using siRNAs conjugated to GalNAc.2
More and more companies are developing their own therapies as well, such as Silence Therapeutics, Arbutus Biopharma, Dicerna Pharmaceuticals, and Arrowhead Pharmaceuticals. Collectively, these companies have a plethora of siRNA-based therapies in both pre-clinical and clinical stages of research, though none have completed a phase III trial as of yet. Quark Pharmaceuticals is moving away from targeting the liver with siRNAs to using them as treatments for kidney injury and eye diseases.4 siRNA-based therapies haven’t been ruled out for cancer either, with two of these companies, Silence Therapeutics and Arrowhead Pharmaceuticals, currently testing their compounds in diseases like myelodysplastic syndrome and renal cell carcinoma. One company, Gradalis Inc., is even utilizing siRNA technology to enhance the immune response to several types of cancers, from Ewing’s sarcoma and colorectal cancer to breast and lung cancer.
Despite recent advances, siRNA therapeutics still have a long way to go. With the recent success of Alnylam’s ONPATTRO and GIVLAARI, it’s only a matter of time before many other RNAi-based drugs hit the market. You might even see some new technologies applied to RNAi-based therapies, like antibody-siRNA conjugates or exosomes used as carriers, all of which could lead to more efficient targeted delivery.4
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- Saw PE, Song EW. siRNA therapeutics: a clinical reality. Sci China Life Sci. 2019:1-16.
- Hu B, Weng Y, Xia XH, Liang X jie, Huang Y. Clinical advances of siRNA therapeutics. J Gene Med. 2019;21(7):1-14.
- Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843-856.
- Setten RL, Rossi JJ, Han S ping. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18(6):421-446.