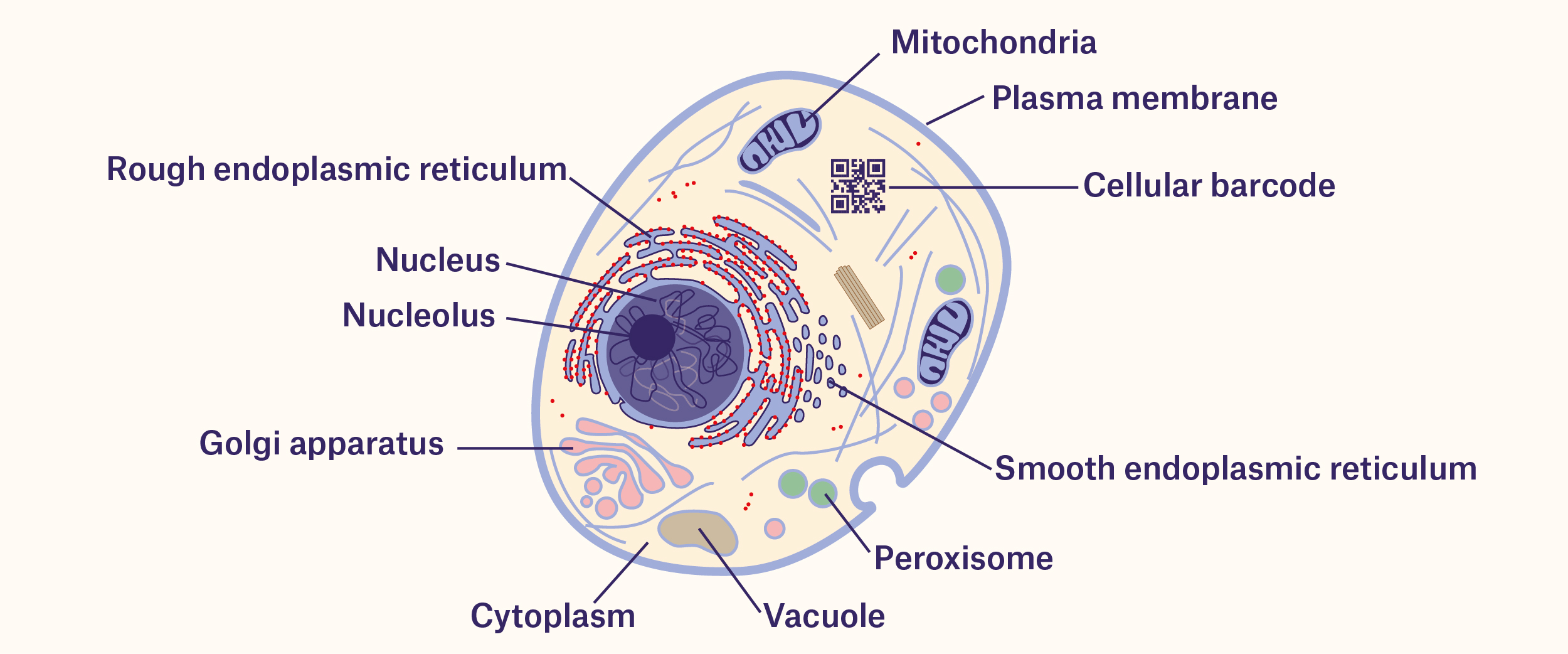
How are cells barcoded?
Cellular barcodes were first generated 25 years ago by manipulating specific stretches of DNA, allowing researchers to effectively tag cells. Currently, the most common method used to barcode cells is by developing a pool of barcoded vectors (e.g. plasmid or viruses) that can be delivered to a population of cells. These vectors would each have enough unique DNA sequences to make it unlikely that two cells would have the same barcode. While this method is ideal for barcoding in vitro (cells cultured in plates and dishes), it is difficult to implement in vivo (i.e. in vertebrate models such as mice and rats) due to the inefficient delivery of the vectors. Several methods have been designed with the goal of improving the efficiency of barcoding cells in vivo, including recombinase- and CRISPR-based barcoding. Recombinase-based barcoding involves using an enzyme, the Cre recombinase, that can recombine DNA sequences surrounded by other specific “recognition” sequences. By repeatedly using the Cre recombinase in conjunction with genes that encode fluorescent proteins (fluorophores), you can generate different combinations of fluorophores to distinguish individual cells. The CRISPR/Cas9 system, used to efficiently edit the genome of any organism, can also introduce mutations in the form of short insertions or deletions at specific cut sites using guide RNAs, allowing each unique mutation to serve as a barcode. Some CRISPR-based barcoding systems even take advantage of the CRISPR/Cas9 system itself. The guide RNAs, produced from the CRISPR/Cas9 vectors that are transfected into cells, contain a spacer element (i.e. DNA sequences not critical for the function of the guide RNA itself) that the CRISPR system can edit, thus conferring a unique, barcoded sequence within each guide RNA molecule.1
Like most barcodes affixed to labels, cellular barcodes also need to be read. Currently, the most widespread method involves analyzing DNA or RNA extracted from the cells by PCR. However, this destroys the cellular architecture being examined. Methods of barcoding that take advantage of fluorescence imaging, like the MEMOIR method of barcoding, which uses fluorescence in situ hybridization (FISH) to probe sequences, can be used to read the cells without damaging tissues or organs.1
What are researchers using barcoded cells for?
The main utility of cellular barcodes lies in their ability to track cells during development. By labeling cells with barcodes, you can follow them as they divide and differentiate, identifying their fate in the body or tracing back their parental lineage. The term “cellular barcoding” was coined by a group who, in 2008, used fate-mapping experiments to characterize T cells in response to immune challenges. Since then, barcodes have been used to identify specific cell populations in high-throughput screenings, to map brain circuits at the level of synapses using individually labeled neurons, and to develop molecular recording devices that can assess the intensity of different stimuli, including inflammatory signals.1
Unique types of cellular barcodes
Genetic barcodes aren’t the only kind of barcodes that researchers have used to label cells with. In 2014, Zhang et al described a novel barcode composed of small rod-like crystals that fluoresce in two colors simultaneously. The rods can have either red, green, or blue tips, which can be adjusted in length to generate distinct barcoded rods. The authors showed that the rods can be internalized by cancer cells, yielding a new potential platform to follow tumor progression and metastasis.2 Sergi Novo and his colleagues at the Universitat Autònoma de Barcelona developed another kind of barcode system more akin to the kinds of barcodes you’d normally find on container labels. His team designed tiny polysilicon wafers that could carry 8 bits of binary information, mixed them with human sperm to tag the cells, then identified the samples using an automated reading system. With no visible effects on sperm quality or quantity, the barcodes could be applied in assisted reproduction labs to ensure that the correct cells are used for all in vitro fertilization procedures.3 Finally, in a 2018 paper that was published in Cell, researchers from the Icahn School of Medicine at Mount Sinai designed a method of barcoding proteins that exploits the CRISPR/Cas9 system. Utilizing triplet combinations of linear epitopes (the portion of a protein that is recognized by an antibody), they were able to synthesize over 100 unique barcode patterns that were introduced into cells using vectors and analyzed. Using a set of 14 antibodies specific to these epitopes, they were then able to identify several phenotypic markers important for cancer immune editing.4
Within the last few years, barcoding technology has become significantly more advanced. With the ability to tag cells with unique identifiers, scientists can better understand how organisms develop and how their cells communicate with one another, as well as optimize screens to discover novel therapeutics to treat human illness. Whether you’re tracking sample containers or the samples themselves, barcoding is important in helping the scientific community solve complex biological and medical problems.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- J.M. Kebschull; A.M. Zador. Cellular barcoding: lineage tracing, screening and beyond. Nat Methods. 2018;15:871-879.
- Zhang Y, Zhang L, Deng R, et al. Multicolor barcoding in a single upconversion crystal. J Am Chem Soc. 2014;136(13):4893-4896.
- Novo S, Mora-Espí I, Gómez-Martínez R, et al. Traceability of human sperm samples by direct tagging with polysilicon microbarcodes. Reprod Biomed Online. 2015;31(2):162-170.
- A.Wroblewska; M. Dhainaut; B. Ben-Zvi; S.A. Rose; E.S. Park; E.D. Amir; A. Bektesevic; A. Baccarini; M. Merad; A.H. Rahman; B.D. Brown. Protein barcodes enable high-dimensional single-cell CRISPR screens. Cell. 2018;175(4):1141-1155.