Recently, a news article on optimizing lab protocols described how several researchers at the University of Montreal decided to devise a new strategy for culturing cortical neurons.1 This article brings to light the ongoing drive of scientists to constantly improve and optimize their protocols; to wit, scientific papers have been published detailing the needs of researchers when optimizing protocols, highlighting cost-effective and robust procedures based on validated models.2 Though some of these models are intricately complex, certain broader themes can help scientists enhance the accuracy and consistency of their scientific procedures, including the need to standardize sample identification.
Standardization applies to every facet of a protocol
The key to any lab protocol is standardization. Without standardization, it is impossible for others to repeat experimental data successfully and to ensure that diagnostic or prognostic testing results are accurate. However, variations often appear from lab to lab within a given workflow based simply on the unique characteristics of a given lab and the reagents/equipment available to them. Regardless, within the lab, once an assay has been tested and shown to work, personnel will devise a standard operating procedure (SOP) in order to ensure results are kept consistent.
When writing an SOP, controlling for as many aspects of the assay as possible is crucial. Everything should be accounted for: reagent storage conditions, volumes and concentrations, specific containers, and incubation times. Unfortunately, the sample tracking method is not always included within SOPs, which also requires its own method of standardization and optimization.
How to optimize sample identification
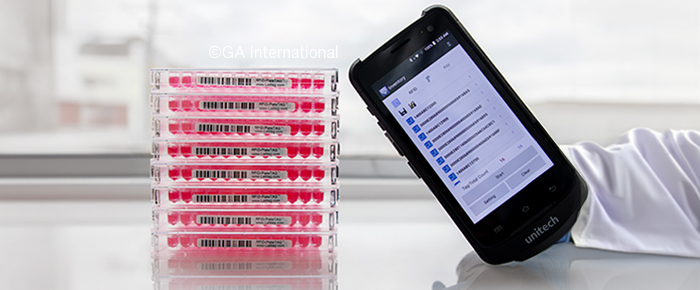
The first step in standardizing and optimizing sample tracking and identification for experimental and analytic protocols involves the use of laboratory digitization. Typically, a laboratory information management system (LIMS) and/or an inventory management system are used as online frameworks to organize and manage inventories of samples and reagents and to track their use during workflows. To accurately and consistently track samples, a labeling system involving barcodes and/or radio-frequency identification (RFID) is implemented, which allows individual containers to be scanned and monitored as they pass from one step to another.
Optimizing such a system requires several steps. First, it is necessary to design an efficient system that matches the needs of the lab’s workflows. The use of barcodes and/or RFID should be based on the volume of samples the lab runs, among other factors. Labs running a high throughput may require RFID tracking, which can scan multiple items simultaneously without a direct line-of-sight. For smaller labs, barcodes may be more cost-effective.
Label types must also be chosen appropriately depending on the lab environments the samples are exposed to. Samples stored in liquid nitrogen or ultra-low temperature freezers require cryogenic labels, whereas those used in histology may need xylene-resistant labels. When deciding to integrate a LIMS, it is essential to assess every type of label needed in order to mitigate errors and fully optimize the system.
Similar to nearly all biomedical workflows, once a sample identification system is selected, testing is required to ensure it works as intended. Partnering with a trusted label manufacturer benefits the user as they can provide samples to test and help troubleshoot along the way as well.
Improved efficiency and sustainability through optimization
With a reproducibility crisis ongoing, lab optimization and standardization are necessary across the board. Time and money spent on performing scientific inquiries is often lost, as others cannot reproduce similar results. Here, lab digitization can play a massive role by providing several benefits to scientists:
- Improved research quality: Standardization results in heightened work integrity and consistency, thus providing greater odds of reproducibility and more concrete scientific study.
- Higher productivity: With standardization, time and labor are saved from constantly reimagining protocols and troubleshooting.
- Cost-effectiveness: Optimizing procedures leads to faster innovation and better use of resources.
Ultimately, all labs should take steps to fully optimize their protocols, including ensuring that sample identification is as accurate and consistent as possible. Through digitization, with LIMS and barcode and/or RFID labels, protocols can be refined, reducing the likelihood of human error and resulting in more accurate short- and long-term results.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
- Khan T. The Problem with Protocols. The Scientist. January 2024. Available at: https://www.the-scientist.com/news/the-problem-with-protocols-71600.
- Flahert P, Davis RW. Robust Optimization of Biological Protocols. Technometrics. 2015;57(2):234-244.


