Carbon capture technology has been around for a while, with new advancements being developed to help combat climate change. Carbon capture and storage is a combination of technologies designed to prevent the release of CO2 generated through conventional power generation and industrial production processes and storing the CO2 in underground reservoirs. However, a recent discovery might allow that captured CO2 into valuable products while addressing climate change.
Existing Carbon Capture Technology
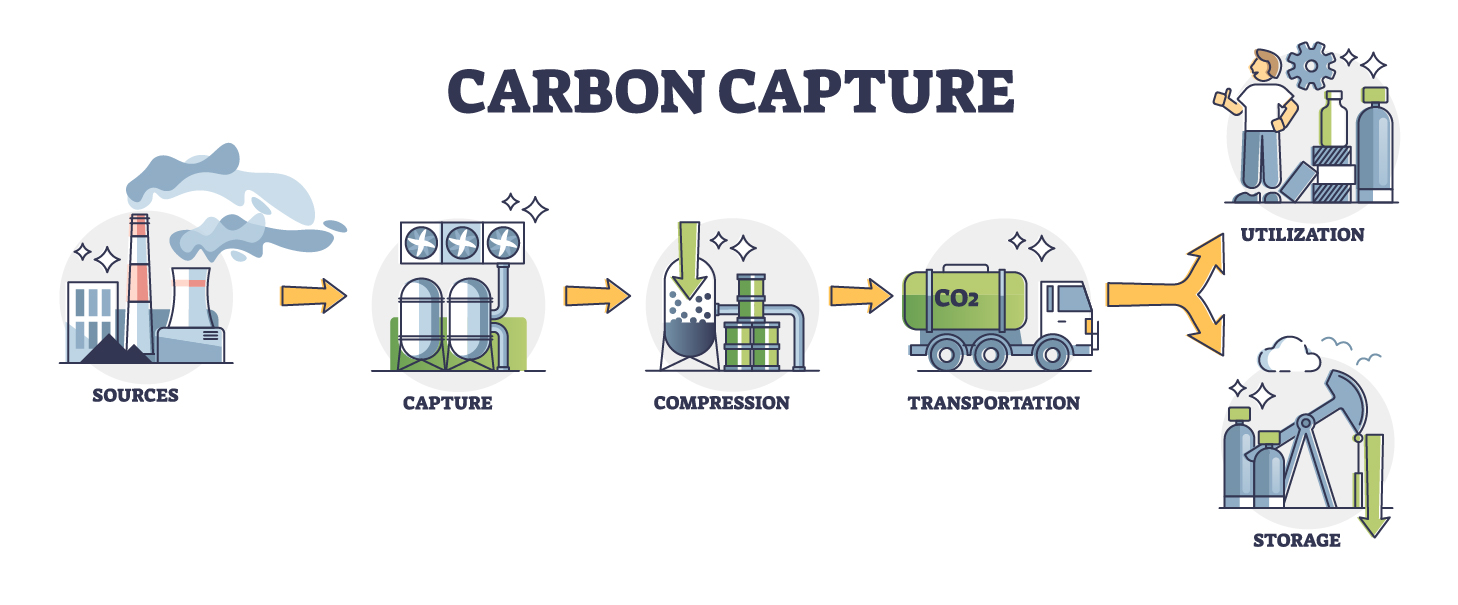
CO2 capture technology separates CO2 emissions from the environment and compresses them for storage in a suitable storage location. The compressed CO2 can be transported via pipeline or by traditional shipping methods. The captured CO2 can then be stored in abandoned oil and gas fields, deep saline formations, as well as no longer mineable coal seams. The primary reason CO2 is needed is to reduce emissions generated by industry and power generation, which would allow fossil fuel use to continue for the time being while still generating a significant decrease in CO2 emissions.
Several technologies are currently being employed in the capture, transport, and geological storage of CO2. Typically referred to as “capture technologies”, they can be grouped into three categories, depending on the industry targeted. Development of these technologies has primarily focused on the efficiency of removing CO2 from the other compounds usually emitted by the industrial process.
- Post-Combustion: Post-combustion methods involve the capture of CO2 from gas streams produced after the combustion of fossil fuels or other carbon-based materials. The post-combustion separation process involves using solvents to separate carbon dioxide out of the gas and capture it. The thermodynamic driving force for this method is relatively low, typically less than 15%. Common applications for this technology include pulverized coal plants and natural gas combined cycle plants.
- Pre-Combustion: This process removes CO2 from fossil fuels before combustion is completed. The fuel in the process is reacted with either steam, air, or oxygen and converted into a mix of carbon monoxide and hydrogen. The carbon monoxide is then converted to CO2 and separated from the hydrogen, which can be used to generate power and/or heat. This process performs slightly better in terms of energy efficiency than post-combustion capture; however, while the energy demand is lower, the capital investment is larger due to the more complex installation.
- Oxyfuel Combustion: The oxyfuel combustion process burns fuel using pure oxygen, or a mixture of oxygen and recirculated gas. This produces a flue containing mainly water vapor and a high concentration of CO2 (80%). Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. The gas is then cooled to condense the water vapor, which leaves an almost pure stream of CO2.
Public Perception & Environmental Impact
With any new technology, there is always some public concern, as well as regulatory uncertainty in its initial stages. The primary concern with carbon capture technology is the storage of CO2 and the potential environmental impact it may cause. While modifications to international legislative provisions have been made recently to accommodate for the offshore storage of CO2, many apprehensions remain concerning storage liability, monitoring responsibility, and the transport of CO2. While general frameworks have been adopted in certain parts of the world, discussions are still ongoing in others, like the United States. According to scientists, this is partly due to the mixed public opinion on the technology, believed to be tied to a general lack of awareness. In fact, in several communities where CO2 storage projects were planned, local stakeholders have shown concern about the risks, leading to protests.
While carbon capture technology and storage can potentially significantly reduce CO2 emissions generated by industrial installations, it still poses a risk to the environment from possible leaks in transportation pipelines or storage sites. CO2 leaking from a pipeline entails similar risks to natural gas leaks, though CO2 is not flammable. Moreover, while not poisonous, it can lead to asphyxiation if the concentration in the air becomes high enough. As such, several countries have established regulatory standards for the transport and permanent storage of CO2. Negative environmental impacts can be associated with the toxicological impact related to the use of solvents to chemically trap the CO2 and the energy penalty required to operate the capture unit.
New Discovery
Engineers at the University of Cincinnati have developed a promising new CO2 conversion procedure that may make the process more economically viable for industries1. The method comprises an electrochemical system that converts emissions from chemical and power plants into useful by-products that can be used to generate new materials. Utilizing a two-step cascade reaction, this new technique converts carbon dioxide first into carbon monoxide and then into ethylene. Ethylene is an important industrial organic chemical that has been called “the world’s most important chemical”1. It’s used in the production of a range of plastics from water bottles to PVC pipe and textiles, to manufacture ethylene oxide, polyethylene for plastics, alcohol, and even rubber found in tires and insulation.
The significance of using a two-stage conversion process is that ethylene selectivity can be increased while also increasing productivity in a process that can be applied to various reactions, given its simple electrode structure. In the future, this technique can be used to reduce carbon emissions while also generating a profit for the industry, eliminating one of the main drawbacks of current carbon capture processes. However, the current process, using copper, requires more energy than it produces in ethylene. While great strides have been made in improving the process, more work still needs to be done, though a superior catalyst than copper would likely solve the economic issue.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
Reference:
- Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Tianyu Zhang, Justin C. Bui, Zhengyuan Li, Alexis T. Bell, Adam Z. Weber, & Jingjie Wu. Nature Catalysis vol5, 202–211 (2022).