 Syringes are one of the most integral tools of any medical institution. Primarily used for injectable medications, they are critical to proper patient care, as anesthesiologists depend on them to sedate and anesthetize. With that in mind, it’s important to consider what pertinent information to include when labeling syringes, which may hold any number of different classes of drugs, such as the name of the drug and its concentration, as mislabeled syringes could yield potentially dire consequences for those who are injected with the wrong substance or dose.
Syringes are one of the most integral tools of any medical institution. Primarily used for injectable medications, they are critical to proper patient care, as anesthesiologists depend on them to sedate and anesthetize. With that in mind, it’s important to consider what pertinent information to include when labeling syringes, which may hold any number of different classes of drugs, such as the name of the drug and its concentration, as mislabeled syringes could yield potentially dire consequences for those who are injected with the wrong substance or dose.
Medical errors resulting from poor labeling during drug administration
Researchers have performed several studies to assess the frequency of medical errors due to mislabeled syringes and to test strategies that may minimize those errors. A review of 896 incidents occurring between 1988 and 2011 from the Australian Incident Monitoring Database found that 50.4% of these incidents were caused by syringe and drug preparation errors.1 One study, performed by Fasting and Gisvold in 2000, described a rate of 0.11% for anesthesia-related drug errors. Out of 63 identified cases, 3 were “serious” to the patients and 27 resulted in “moderate” unwanted effects. In this study, they contributed the bulk of these errors to mixing up syringes with the same size or solution color, while noting that muscle relaxants were frequently misused, leading to partial or full temporary paralysis of the patient and, ultimately, postoperative anxiety.2 Nearly 11 years later, errors in drug administration were still prevalent, as Alan Merry et al found a rate of 0.33 drug administration errors per 100 administrations using conventional methods of preparing and labeling syringes, with an additional 12.10 recording errors documented per 100 administration.3
Labeling guidelines to help medical practitioners make fewer errors
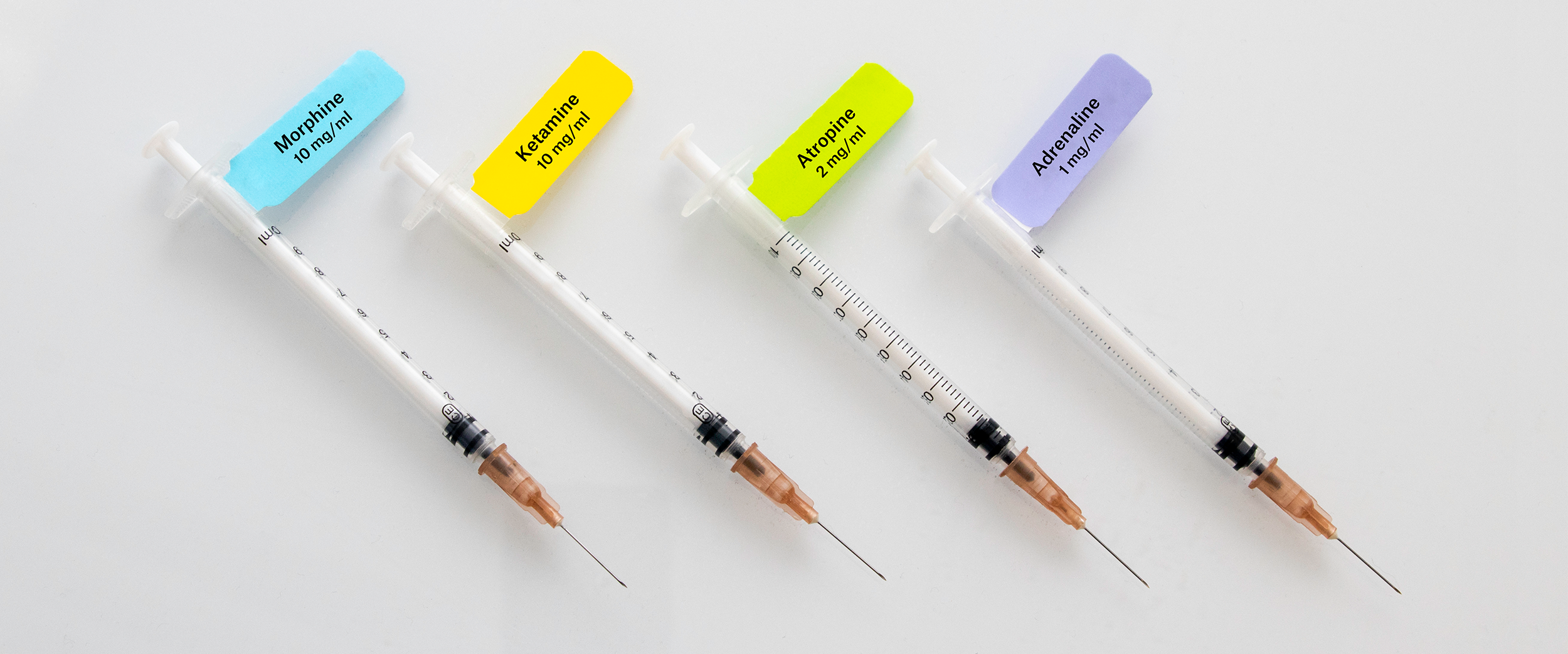
To help make drug administration safer throughout medical institutions, guidelines have been published detailing recommendations for syringe labeling. The most prominent set of guidelines was published in 2008 by the International Organization for Standardization (ISO). They recommend stringent requirements for user-applied syringe labels, including:4
- The use of self-adhesive labels that can be applied to polyethylene syringes and can withstand isopropanol exposure
- The printing of the drug name on the upper half of the label
- The use of diagonal stripes when indicating a drug of opposite action
- The provision of space to hand-write additional information, like drug concentration
- Lower-case lettering with an initial upper-case letter
- In certain cases, the use of upper-case letters for distinguishing parts of similar drugs names, otherwise referred to as “tall-man lettering” (e.g. using CARBOplatin and CISplatin instead of carboplatin or cisplatin)
- Specific color specifications for each drug class (outlined in Table 1)
ASTM International, formerly the American Society for Testing and Materials, has a similar set of guidelines for syringe labeling, with a few additional recommendations such as using a maximum amount of contrast between the text and the background of the label to minimize errors due to color blindness, color coding for beta blockers, and barcoding, which forces the administrator to manually scan each syringe before use, ensuring that the correct drug has been chosen.5 Other organizations around the world, such as the Austrian and New Zealand College of Anesthetists (ANZCA) and the French Society for Anesthesia and Critical Care (SFAR), have their own syringe labeling requirements; however, most generally resemble the guidelines set by ISO.6,7
Often, drugs are prepared directly from vials or ampoules then placed into a syringe, leading to a situation where the information about the drug name and dose is lost. In these cases, the US Food and Drug Administration suggests that these commercial containers come with transferable or peel-off labels, so that they can be attached directly to the syringe at the point of preparation, allowing the user to transfer all pertinent information immediately.8
Table 1. Pantone color matching system used in ISO and ASTM International guidelines for syringe labeling.4,5
|
Drug class |
Pantone color |
RGB code |
Induction agents |
Process yellow C |
255.255.0 |
Benzodiazepines & tranquilizers |
Orange 151 |
255.102.0 |
Benzodiazepine antagonists |
Orange 151 + white diagonal stripes |
255.102.0 + white stripes |
Muscle relaxants |
Fluorescent red 805*/811†, warm red |
255.114.118, 253.121.86, 245.64.41 |
Muscle relaxant antagonists |
Fluorescent red 805*/811† + white diagonal stripes |
255.114.118, 253.121.86 + white stripes |
Opioids & narcotics |
Blue 297 |
133.199.227 |
Opioid & narcotic antagonists |
Blue 297 + white diagonal stripes |
133.199.227 + white stripes |
Major tranquilizers & anti-emetics |
Salmon 156 |
237.194.130 |
Vasopressors |
Violet 256 |
222.191.217 |
Hypotensive agents |
Violet 256 + white diagonal stripes |
222.191.217 + white stripes |
Local anesthetics |
Gray 401 |
194.184.171 |
Anticholinergic agents |
Green 367 |
163.217.99 |
Beta Blockers‡ |
Copper 876U†, White |
176.135.112, 255.255.255 |
*ISO guidelines only
|
||
Quality care depends on rigorous labeling standards. With so many different drugs used throughout medicine, injecting the wrong one, whether it’s an anesthetic, analgesic, or even a vaccine, can have serious consequences for the patient. For anesthesiologists and all other medical professionals performing injections, having a clear, identifiable labeled syringe could mean all the difference, especially when there isn’t always a lot of time to spare.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- Velayudhan S, Arumugam V. Syringe label: A potential source of dosage error. Indian J Anaesth. 2014;58(4):506–507.
- Fasting S, Gisvold SE. Adverse drug errors in anesthesia, and the impact of coloured syringe labels. Can J Anesth. 2000;47(11):1060–1067.
- Merry AF, Webster CS, Hannam J, et al. Multimodal system designed to reduce errors in recording and administration of drugs in anaesthesia: Prospective randomised clinical evaluation. BMJ. 2011;343:1-14.
- International Organization for Standardization. International Standard (ISO26825): Anaesthetic and Respiratory Equipment — User-Applied Labels for Syringes Containing Drugs Used during Anaesthesia — Colours, Design and Performance. Geneva, Switzerland; 2009.
- American Society of Anesthesiologists. Statement on Creating Labels of Pharmaceuticals For Use in Anesthesiology. Schaumburg, Il; 2015.
- Piriou V, Theissen A, Arzalier-Daret S, et al. Preventing medication errors in anesthesia and critical care (abbreviated version). Anaesth Crit Care Pain Med. 2017;36:253-258.
- Australian and New Zealand College of Anaesthetists (ANZCA). Guidelines for the Safe Management and Use of Medications in Anaesthesia. Melbourne, Australia; 2018.
- US Food and Drug Administration (FDA). Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors. Silver Spring, MD; 2013.



