 Science has recently begun to establish some of the tools that might let us develop a form of synthetic life. Developing cells from scratch ought to let us understand a whole lot more about what actually constitutes a living organism while making it possible to generate simpler (yet no less sophisticated) life-like organisms that can be more predictably manipulated.1
Science has recently begun to establish some of the tools that might let us develop a form of synthetic life. Developing cells from scratch ought to let us understand a whole lot more about what actually constitutes a living organism while making it possible to generate simpler (yet no less sophisticated) life-like organisms that can be more predictably manipulated.1
Top-down approach to synthetic biology
There are two approaches that scientists have employed to attempt to make synthetic cells: the top-down approach and the bottom-up approach. The top-down approach involves taking a simple system, like a bacterial cell, and injecting genetic material into it, causing it to act or react differently than it normally would. Though more complex than strategies used to modify single genes, this approach uses similar methods to add foreign elements, or “modules”, to existing cells. For example, yeast—single-cell eukaryotes—have been engineered to produce artemisinic acid, a key precursor of an anti-malarial medication that was once in short supply. Another example includes replacing the entire genome of one species with the genome of another.2
Bottom-up approach to synthetic biology
The bottom-up approach works by creating an entirely new organism from scratch instead of using the “shell” of an organism as a base. This has yet to be fully accomplished, as the complexity of bringing together multiple functioning parts, from membranes to genetic information, makes it extremely hard to produce a functional unit of life.2 So, if you were to create an organism from scratch, what exactly would you need? There are several aspects of life that need to be considered before we can generate life from organic building blocks:
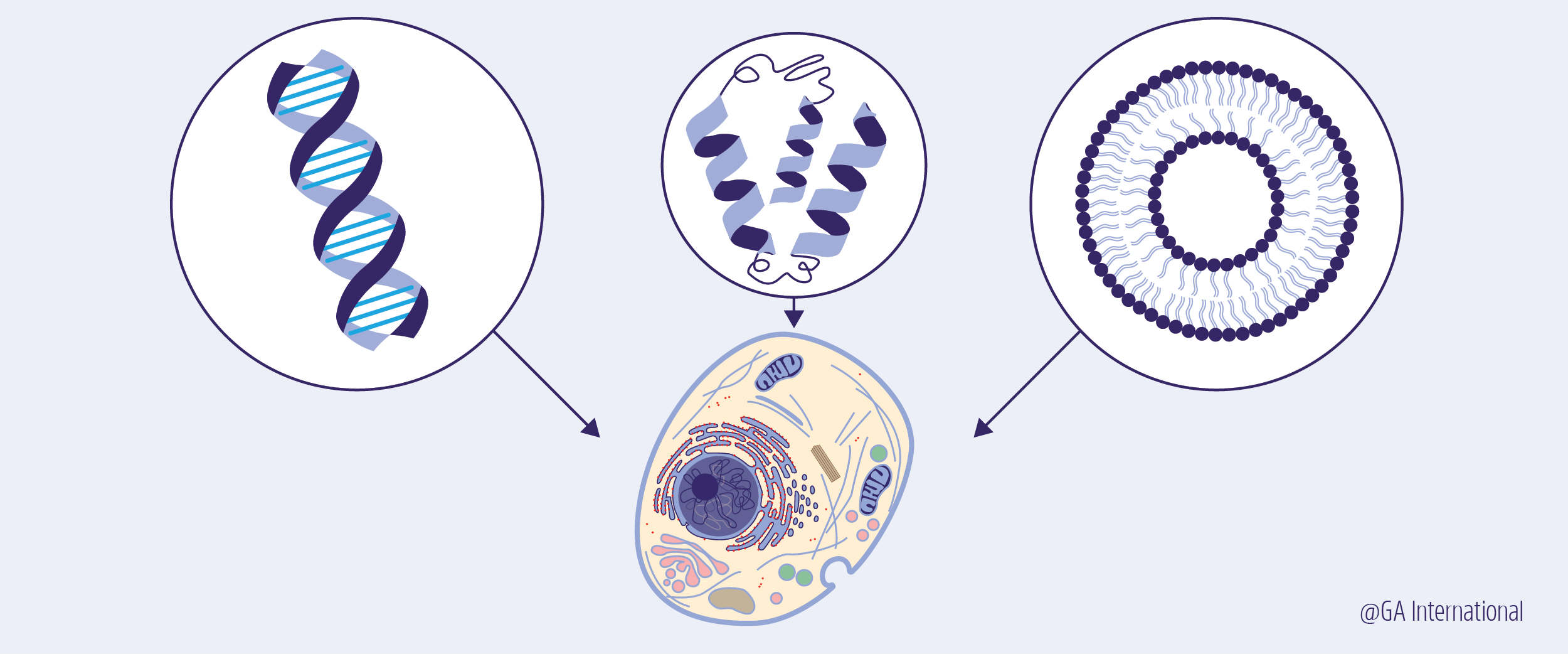
Compartments – What defines an organism is a membranous compartment that allows it to sustain life. However, membranes are dynamic in nature, letting certain elements into the cell, keeping others out, and communicating with the environment. A newly synthesized membrane ideally performs all these functions, with just the right amount of permeability to eliminate waste but retain necessary cellular components. The factors that need to be considered when designing membranes for synthetic cells include the chemical nature of the membrane as well as its thickness, pores, channels for molecules to pass through, and domains (parts of the membrane that are specialized to perform specific functions).3 To develop a new eukaryotic cell, an endomembrane system also needs to be put into place, with multicompartment vesicles physically separating the many functions the cell will need to carry out.4
Genetic programming – Synthetic life will also need some form of information system to pick and choose what kind of proteins need to be built at any given time. As DNA is used in every type of cell on Earth, it will also be the most likely source of genetic information in newly synthesized cells.3 It might also be important to consider how the information is processed in synthetic cells, as gene expression must be responsive to extracellular cues signaled to the processing center of the cell (i.e. the nucleus).5
Energy and metabolism – To maintain activity, cells require energy. In most cells, the main source of energy is supplied in the form of ATP, which yields high-energy bonds that can be broken down to provide the resources necessary to accomplish any number of tasks. A simplified metabolic pathway that generates ATP and other metabolites, which can be used for downstream reactions, is likely ideal for a newly created synthetic organism.5 This requires some form of input, either a carbon source, light source, or both, alongside a way for the organism to recycle and/or excrete waste in order to prevent metabolism from halting prematurely and to limit the accumulation of toxic byproducts.3
Growth and division – The generation of new membranes is a necessary feature that allows cells to grow and divide. New membranes prevent the organism from shrinking and make it possible for a cell to divide into 2 equally sized daughters.3 The genetic material must also be replicated so that each daughter cell receives the same information, a factor so critical that life could not have emerged 4 billion years ago without it.1 To do so, a system with an ability to alter the shape of its own membranes (similar to the cleavage furrow of mitosis) and to spatially organize itself must be integrated so that it sustains not just the replication and division of genetic material, but also the metabolism of the cell after division.3,5
Adaptivity – Finally, the synthetic cell must have a way of diverting resources towards increasing its chances that it will encounter food. For many cells, this means some form of locomotion, whether it be a tree that grows towards the light or the cilia on a bacterium that allow it to swim towards nutrients. The cell must also have a way of sensing the nutrients around itself and signaling to its motile elements of choice that they need to activate in order to search and locate food.5
Recent breakthroughs in creating artificial cells
Several groups have been able to create a limited version of synthetic life using the bottom-up approach. In 2015, a group led by Kensuke Kurihara from Japan developed a model using giant vesicles (GVs) to produce “protocells” that can replicate themselves for multiple generations. In their model, they designed membranes composed of a limited number of phospholipids that encapsulated DNA, which could altogether be divided symmetrically when a catalyst was added to the cells. They showed that this system could self-propagate for at least 3 generations.6 Later, in 2017, Lentini et al reconstituted a quorum-sensing pathway (i.e. a pathway that lets the cell sense the density of microorganisms surrounding it) in artificial cells, allowing them to communicate with natural bacteria.7 In 2018, a group in California discovered that oleoyl β-D-1-thiogalactopyranose (OTG) could spontaneously form micron-sized vesicles that, when supplied with genetic material, could successfully amplify DNA, with a membrane pore large enough to let dNTPs (the building blocks for DNA) into the cell.8 This same group also made their synthetic cells efficiently express a gene for green fluorescent protein (GFP) by simply adding the necessary components to the medium surrounding the cells. To add complexity to their system, they added cells that could not produce the GFP signal themselves but could release a molecule that turned on GFP signaling in their neighboring cells instead, allowing two kinds of artificial cells to effectively communicate with each other.9
Recently, strides have been made to expand upon different aspects of synthetic biology. Petra Schwille and her team in Germany have been studying the effects of incorporating Min proteins, which coordinate cell division in bacteria, into lipid membranes, causing them to contract in the center, as if they’re undergoing division. Another group in Germany led by Joachim Spatz has designed an artificial mitochondria-like organelle that can generate ATP in a vesicle, while Tobias Erb, also researching synthetic biology in Germany, has developed a system to convert CO2 into useful metabolites inside artificial bioreactor-like cells. Finally, in an example of how top-down synthetic biology affects the bottom-up approach, John Glass and his team in California synthesized a minimal artificial genome called JCVI-syn3.0a, using only genes that were necessary for the growth and development of Mycoplasma capricolum, and replaced the genome of another type of bacteria with it. As the new genome was able to keep the bacteria thriving, the group now hopes that this synthetic genome could be used to start up artificial cells from scratch.10 Finally, a group from the Tokyo Institute of Technology have constructed artificial photosynthetic cells that use a light-harvesting protein, bacteriorhodopsin, to produce ATP.11
With initiatives like MaxSynBio, a research consortium out of the Max Planck Institute in Germany, as well as Building a Synthetic Cell (BaSyC) in the Netherlands, research into creating artificial cells is alive and well. Funding for these projects is substantial, as BaSyC is working off of a $21.3 million Dutch Gravitation grant, with another $10 million from the US National Science Foundation (NSF) being given to scientists studying artificial life.1,10 BaSyC has set a goal of 10 years for its members to produce the first-ever cell built from scratch.10 With science coming closer and closer to developing functional synthetic components each year, it might become a reality even sooner.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- Schwille P, Spatz J, Landfester K, et al. MaxSynBio: Avenues Towards Creating Cells from the Bottom Up. Angew Chemie – Int Ed. 2018;57(41):13382-13392.
- Max-Planck-Gesellschaft. Synthetic Biology: Life, Remixed. https://www.mpg.de/8219292/synthetic_biology. Published 2014.
- Buddingh’ BC, Van Hest JCM. Artificial Cells: Synthetic Compartments with Life-like Functionality and Adaptivity. Acc Chem Res. 2017;50(4):769-777.
- Göpfrich K, Platzman I, Spatz JP. Mastering Complexity: Towards Bottom-up Construction of Multifunctional Eukaryotic Synthetic Cells. Trends Biotechnol. 2018;36(9):938-951.
- Yewdall NA, Mason AF, van Hest JCM. The hallmarks of living systems: towards creating artificial cells. Interface Focus. 2018;8(5):1-15.
- Kurihara K, Okura Y, Matsuo M, Toyota T, Suzuki K, Sugawara T. A recursive vesicle-based model protocell with a primitive model cell cycle. Nat Commun. 2015;6:1-7.
- Lentini R, Martín NY, Forlin M, et al. Two-Way Chemical Communication between Artificial and Natural Cells. ACS Cent Sci. 2017;3(2):117-123.
- Brea RJ, Bhattacharya A, Bhattacharya R, Song J, Sinha S DN. Highly Stable Artificial Cells from Galactopyranose-Derived Single-Chain Amphiphiles. J Am Chem Soc. 2018:1-6.
- Leslie M. Biologists create the most lifelike artificial cells yet. Science (80- ). November 2018:1-13.
- Powell K. How biologists are creating life-like cells from scratch. Nature. November 2018:1-13.
- Berhanu S, Ueda T, Kuruma Y. Artificial photosynthetic cell producing energy for protein synthesis. Nat Commun. 2019;10(1):1-10.