Did you know that a simple change in the design of medication bag labels such as IV or blood bags can decrease medication errors? Labels with clear and easy-to-read printed data can be the difference between administering the right or wrong drug(s) to a patient. Furthermore, specific regulations and recommendations on identification must be followed to meet national standards for safety.
The five “rights” method & understanding medication bag labeling
Labeling medication bags is a key part of the “five rights” method for safe medication practices. This entails administering the right medication, in the right dose, at the right time, by the right route, and to the right patient1. Line or drug infusion bag labels help manage the flow of medication into intravenous (IV) lines and that patients receive the correct dose when they need it. As such, these labels must include certain information, including:
- Patient Name
- Room Number
- Solution Information
- Preparation Time & Date
- When Infusion Started
- Any added Drugs & Strengths
- Expiration Date
Placing the labels at the top of the line or near the insertion point will also help ensure proper dispensing, while noting the dose and strength of the medication on the bag guarantees consistent administration, even during personnel shift changes. Moreover, noting the start and end times assures the patient receives the proper dosage. Color labels can also be used on various lines to help staff verify that lines are correctly connected.
The type of bag being labeled can also affect the specific bag label used, as well as the printed information. The most commonly used type is likely the IV bag label. Used to identify saline solutions administered to patients, these labels must also be updated when additional medications are added. IV bag labels may also require resistance to extreme heat or cold temperatures, as well as abrasion and fading. These labels help ensure staff knows what is currently in the bag, avoiding dangerous mix-ups when medication needs to be added to the solution.
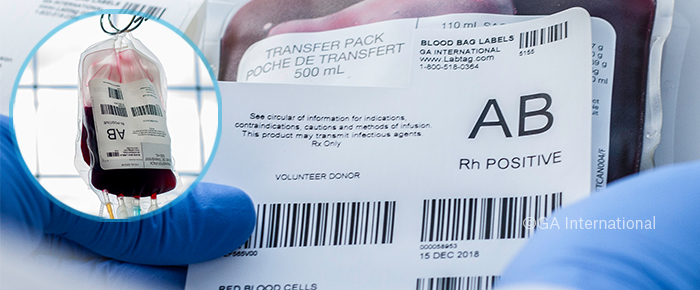
Blood bag labels come in two types, primary and secondary. Primary blood bag labels must comply with stricter guidelines and will typically display information on the manufacturer, as well as batch numbers and product codes for tracking and traceability. Secondary labels are applied over the existing primary label, and as such, are under fewer regulations. These labels can include information regarding blood type and various blood components, such as platelets, plasma, and red blood cell count. Medication bags are often used to administer cancer therapies, including chemotherapy and immune cell (T-cell) therapies. These medication bags will typically come pre-prepared and labeled by the bag manufacturer and may require specialized labels, depending on the composition of the bag and administered medication. Custom labels for bags may need to be produced to meet the bag manufacturer’s particular requirements.
Guidelines & regulations for medication bag labels
The primary concern for the design and use of labels for drug infusion bags should be reducing medication errors to render patient care safer. Medication infusion bags can be labeled by the manufacturer, with additional labels applied by point of care staff located in hospitals and clinics. Different standards and regulations may apply for each of these situations. In either case, the total volume of the bag and the generic name and dose of each drug added should be the most prominently displayed information. The other information mentioned above should also be included, and the label material must permit users to write additional information using ball-point pens or markers2. They can also follow the guidelines for writing drug names, including color and capitalization, though this is more commonly used when identifying syringes.
Medication bag labels must meet the regulations established by ASTM International (formerly the American Society for Testing and Materials), the International Organization for Standards (ISO), as well as recommendations and guidelines from the Food and Drug Administration (FDA) and the Institute for Safe Medication Practices (ISMP). This includes FDA 21 CFR 175.105 for indirect food contact and ISO 26825:2008 regarding label material. These infusion bag labels must also follow FDA regulations for barcoding medication containers. They should include a barcode associated with the generic drug’s name and strength at a location on the label, which will not interfere with its legibility. The use of barcodes is a great way to reduce administration errors by creating an electronic record and decreasing staff workload.
Reducing errors and improving safety with medicine bag labels
The proper use of medication bag labels is invaluable in ensuring safer patient care and that the correct medication is always delivered. However, currently, the design of these labels has raised concerns with staff regarding their use. Some of the concerns raised were poor readability and use of color, as well as a lack of differentiation between similarly-named drugs. Another concern is that the labels can contain information relevant to various stakeholders, resulting in labels that contain an overabundance of information, making finding specific information difficult. This is also the case with IV bag labels that are often shared between pharmacies and nurses. This leads to labels with information required by pharmacists intermixed with information that nurses need for administering the order.
Bauer and Guerlain set out to evaluate the content of IV bag labels and suggested some improvements that can be made in order to enhance patient safety1. They suggested two possible label redesigns to improve the label’s readability. Some of the recommendations they suggested were improved printout, which could be affected by low toner, presenting medication information in a consistent location, and improving readability through capitalization and bold font. The use of thermal-transfer printable labels can help resolve some of the issues raised here, especially regarding the printout. Thermal labels are also well suited for storage and refrigeration, will not smudge, and are perfect for use in various environments.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- Improving the usability of intravenous medication labels to support safe medication delivery. David T. Bauerand Stephanie Guerlain. Int J Ind Ergon. 2011 Jul 1; 41(4): 394–399.
- Statement on Labeling of Pharmaceuticals Used in the Practice of Anesthesiology. American Society of Anesthesiologists. December 13th, 2020.




