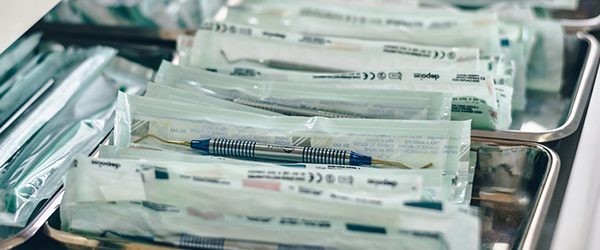
Infection Control and Prevention (IPAC) Canada and the Centers for Disease Control and Prevention (CDC) have stringent guidelines on the sterilization and logging of equipment used in dental offices, required to keep a dental practice compliant. Among the requirements, any material that’s registered as a “critical” or “semi-critical” item, which includes surgical instruments, mouth mirrors, amalgam condensers, reusable impression trays, and anything else that contacts mucous membranes or non-intact skin, needs to be sterilized.1,2
Ensuring all materials are fully sterilized
The implementation of strict guidelines for the sterilization of equipment used in dentist offices is necessary to reinforce the disease and infection control practices currently in place. This includes several common-sense measures, such as not coming to work if sick with an infection and routinely cleaning frequently touched surfaces. For materials that require sterilization, the most common method is steam autoclaving. However, it’s not enough to merely sterilize materials and expect them to be germ-free; an indicator that confirms whether the sterilization process was completed must also be included.
As such, a primary requirement from the CDC and IPAC is that all materials that are sterilized need to have mechanical, chemical, and biological indicators to ensure complete sterilization. Mechanical indicators include the gauges or displays of the machine that indicate temperature, pressure, and cycle time. Biological indicators comprise spore tests that can be included in the cycle to ensure hard-to-kill microorganisms (e.g. Geobacillus or Bacillus species) are eliminated. Finally, a chemical indicator is needed.1,2 There are 6 different types of chemical indicators that can be used:3
- Type 1: Known as Process Indicators, these indicators are applied externally and show when an item has been fully subjected to sterilization.
- Type 2: These indicators are used for certain types of sterilization tests, where the standards require a Type 2 indicator (e.g. Bowie Dick test, which is used to assess the air removal efficiency and steam penetration in steam sterilizers with a pre-vacuum cycle).
- Type 3: Known as Single-Variable Indicators, these respond to a single critical sterilization parameter, such as temperature, pressure, or sterilization time, ensuring the package has been exposed to that parameter during the cycle.
- Type 4: These are Multi-Variable Indicators, which respond top two or more critical sterilization parameters.
- Type 5: Known as Integrators, these respond to all critical parameters of the sterilization cycle. The performance of Type 5 indicators correlates to that of biological indicators.
- Type 6: Known as Cycle-Specific Indicators because they respond to all critical parameters of the specific sterilization cycle that’s been chosen.
The IPAC guidelines state that a Type 1 indicator, usually in the form of either tape or a label, should be placed outside each package along with a Type 4, Type 5, or Type 6 indicator inside. Note that autoclave labels are also typically used to identify vials containing the biological indicator. 1,2
Securing the identity of your materials
The sterilization process involves packaging equipment and consumables and applying intense heat and pressure to eliminate contaminating microorganisms like viruses, bacteria, and fungi. Each item needs to be appropriately identified, ideally with barcoded labels to track every piece as it’s sterilized. However, general purpose labels will fail under extreme conditions, like those of steam autoclaves. Therefore, it’s strongly recommended to use autoclave-resistant labels, which can resist temperatures as high as 150°C and pressures as high as 30 psi. Fortunately, autoclave-resistant labels are currently available for laser, inkjet, and thermal-transfer printers, allowing you to use the printer of your choice. These labels will not only remain firmly affixed to your containers and equipment, but their printout will also not smudge or fade, even if they undergo several cycles of sterilization, ensuring your material remains securely identified.
Keeping a record of sterilization
The recommendations emphasize the importance of having one person in every dental practice assigned to ensure that all guidelines are being followed. One of the primary goals of this individual is to certify that detailed records are kept for all sterilized material. This is because detailed logs must be kept for auditors, who verify your dental practice is compliant and is staying in line with sterilization regulations. All daily activities need to be logged and reviewed in a logbook, ideally using an automated process that tracks all items sterilized and records them electronically.1,2 Some systems, like the ones offered by SteriLog, can print labels as well as log and certify the results from all your indicator tests after each sterilization cycle, including biological indicator and chemical indicator tests.
Sterilization is one of the many things that dental practices need to consider when managing daily operations. However, it doesn’t have to be a burden if you’re using the right equipment. Implementing protocols that utilize the right labels and logging system can save time and prevent errors, keeping your dental practice compliant, and ensuring the highest standards of safety for your dental health care workers and patients.
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
References:
- Center for Disease Control and Prevention (CDC). Summary of Infection Prevention Practices in Dental Settings. Atlanta, GA; 2016.
- Royal College of Dental Surgeons of Ontario. Standard of Practice: Infection Prevention and Control in the Dental Office. Toronto, ON; 2018.
- Steris Healthcare. What is a Chemical Indicator? https://www.steris.com/healthcare/knowledge-center/sterile-processing/what-is-a-chemical-indicator.cfm. Published 2018. Accessed November 6, 2019.